Medical Device Identifiers – Can Safety Wait?
Hurry up and wait appears to be the word from the U.S. Food and Drug Administration (FDA) when it comes to the safety of medical devices.
The agency was supposed to have issued a final proposal by the end of June on a mandate to make it easier to identify medical devices and their problems after they are in use by consumers.
The Unique Device Identification (UDI) system was approved by Congress in 2007 and require the FDA establish a UDI system. The final rule was supposed to be issued within six months of the end of the comment period for the proposed rule. However, the proposal is still under “administrative review,” according to Modern Healthcare.
Med City News reports the Office of Management and Budget (OMB) received a letter last week from the Advancing Patient Safety Coalition (APSC) urging the UDI system be established to improve patient safety calling it “critical to achieving patient safety improvement initiatives and medical error reduction.”
As it stands now, industry would have to implement UDI labeling and a database would be created known as Global Unique Device Identification Database (GUDID). These changes would be phased in over five years and require information on all Class III, II and I devices. The safety group feels that is too long to wait for safety and it urges an expedited implementation of UDI and a shortened timetable.
The group made up of members such as AARP, Association of American Medical Colleges, American Medical Association and the American Nurses Association, among others, urges implementing a one-year timetable for Class III or the most dangerous medical devices. Industry would like that to take two years.
Stryker and DePuy metal-on-metal hips and cardiac defibrillator leads were among medical device recalls in recent years and last year there were 50 medical devices recalled because they were defective and dangerous to patients. A UDI system would help identify problems sooner rather than later before they are used on or implanted in thousands of U.S. patients.
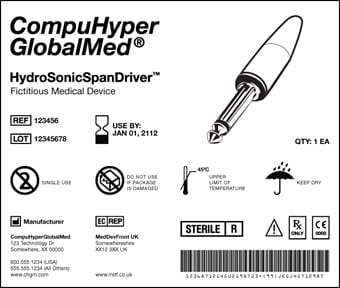
Under the UDI system, the labeling, and in some cases, the medical device itself, will carry a code with the product’s make and model as well as other relevant information including its lot and expiration date.
Part of the holdup appears to be the device manufacturers themselves. AvaMed, which is the the medical device lobby, says on its website that it supports the FDA’s effort to establish the UDI system. It also says the group is working with the FDA “and other stakeholders” to get the system going, presumably to favor industry.
AdvaMed is known to have an enormous control over the FDA and the group claims it needs more time to get ready for the UDI system and question how the system will help the public stay safe.
The group says “burdens should be minimized” on industry, including the financial impact on manufacturers. The FDA has estimated the rule will cost about $514 million over the next 10 years.
Share This