Essure Has Caused 303 Fetal Deaths, Independent Analysis Shows
A gross discrepancy in the number of fetal deaths attributed to the birth control device Essure has prompted the U.S. Food and Drug Administration to analyze the data and decide whether to change the way the product is marketed.
The agency has reported five fetal deaths – along with the deaths of four women implanted with Essure – in 14 years. It first was introduced in 2002 as the only permanent method of birth control that did not require surgery.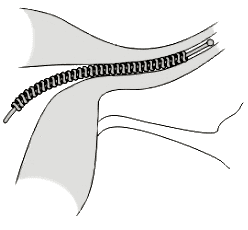
An independent report by a former FDA consultant and employee reveals 303 fetal deaths. Madris Tomes, who heads Device Events, a company focused on obtaining accurate, intelligible date on medical equipment, explained why.
“When adverse events go to the FDA, ‘death’, ‘injury’ or ‘malfunction’ are the boxes you check,” Tomes said in article on Fox News Health titled “FDA likely underestimated fetal deaths from Essure: analyst.” “My system searches the (fuller) narrative.”
Her algorithms use keywords including “fetal death,” “miscarriage,” “stillbirth” and “stillborn” when searching the Adverse Event Reporting System, or FAERS, as the FDA calls it.
One such report dated Aug. 26, 2013, describes a patient who was taken to the emergency room with abdominal pain and, after being examined by the doctor, was diagnosed with infected and necrotic fallopian tubes. Essure, which consists of metal coils, is placed on the fallopian tubes to form a barrier that prevents sperm from reaching eggs.
The woman went into kidney failure and died.
The issue has incensed consumers who have threatened a hunger strike in protest of what they believe is a defective product and launched a Facebook page for those who filing lawsuits. It also has incensed a congressman who introduced legislation to ban Essure if the FDA doesn’t.
“…I request that the FDA conduct a thorough review of this document and all of the adverse event reports received by those harmed by Essure as part of FDA’s on-going review of this medical device,” Republican Pennsylvania Rep. Mike Fitzpatrick wrote in a Feb. 16, 2016, letter to the FDA
Share This


