Avandia and Actos Linked to Macular Edema Vision Problems
According to a study published June 11, the users of Actos (pioglitazone) or Avandia (rosiglitazone) may face an increased risk of developing macular edema. Published in the Archives of Internal Medicine, British researchers collected data on more than 100,000 patients with type 2 diabetes who were enrolled in the British Health Improvement Network database. Actos and Avandia are also called thiazolidinediones that were linked in this study to something called diabetic macular edema, a condition where the macula section of the eye, responsible for sharp vision, swells with a buildup of fluid. It’s unclear whether the condition of diabetes may have contributed to the condition since about 20% of diabetics have macular edema, but participants taking the drugs had a two-to-three fold increased chance of developing macular edema.
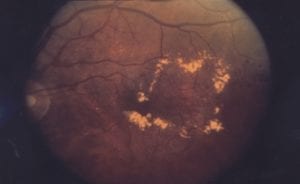
Macular Edema Scan
Still the risk is small University of Nottingham researchers found, about 0.2 percent so the absolute risk is less than 1 percent. The conclusion is that routine vision screening accompanies a diabetes checkup among those patients taking Actos or Avandia.
This is just the latest problems for thiazolidinediones medications.
A British Medical Journal (BMJ) article on Actos by Canadian researchers found that Actos was associated with an increased risk of bladder cancer.
Recently released French and U.S. studies also found a link to an increased risk of bladder cancer as the cumulative dosage increased. And for patients taking 28,000 mg or more of Actos for two years or longer, the relative risk of bladder cancer was increased 88 percent to 137 cases per 100,000 patients or 89.4 cases per 100,000 population years.
Consumer Reports says Actos, made by Takeda Pharmaceuticals, is linked to congestive heart failure, bone fractures, and bladder cancer and is a danger to all patients. If you are taking the diabetes medication, the publication suggests you ask your doctor if there are safer drugs that would do the job such as metformin, glipizide or glimepiride, which have all been around longer and have fewer consumer complaints.
Searcy Denney was one of the first Mass Tort Attorneys to file a lawsuit on behalf of a man who took Actos and developed bladder cancer. His case is among the hundreds consolidated in the U.S. District Court in the Western District of Louisiana.
Meanwhile there are more than 2,500 Avandia lawsuits consolidated in the Eastern District of Pennsylvania in federal court. All of the plaintiffs say if they had just been told about the complications they would have saved their lives and taken some of the older, more reliable diabetes medications.
Share This


