What if you became a human radiation storage facility?
CardioGen-82: Is it business as usual winning over Safety?
Between February 17th and February 21st, 2011, two heart patients, one from Sarasota, Florida and the second from Nevada underwent nuclear PET (Positron Emission Tomography) scans to diagnose possible heart malfunctions. After having undergone CardioGen-82 PET scans, the two patients and at least one other had a lingering problem, residual high level radiation. And if it were not for heightened vigilance from Homeland Security personnel, those patients might never have learned that they were walking radiation repositories.
After the March earthquake that devastated Japan and wreaked havoc on its nuclear facilities, Homeland Security began screening international travelers to detect any possible abnormal radiation levels. On June 3, 2011 while going through a Canadian checkpoint on his way to Buffalo, New York, the first CardioGen patient lit up Homeland Security’s gamma radiation detector. Homeland Security personnel then detained that individual, and he became marooned in Canada for an indefinite period. As a result, he called Heart Specialists of Sarasota, where he had undergone the CardioGen diagnostic PET scan to determine if personnel there could help him to obtain permission to go home.
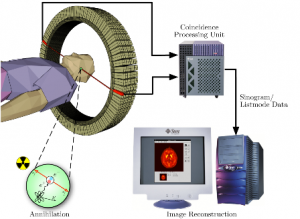
The CardioGen-82 makes tracer in the following way. The daughter element, Rubidium-82 (half-life, 76 seconds) is made within the CardioGen itself from a parent element called Strontium-82 (half-life approximately 4 weeks). Bracco obtains the heart of the CardioGen, a Strontium-Rubidium generator, from Nordion, a Canadian company and one of a few companies in the world that is capable of producing the Strontium-Rubidium generator.
The Strontium-Rubidium generator itself is made of a tube containing a substrate of tin oxide. Nordion then impregnates the tin oxide substrate with the more radioactive Strontium-82. Over a period of about a month, the impregnated Strontium-82 decays and continuously produces the less radioactive Rubidium-82 as a by-product. The CardioGen, equipped with a series of valves, syringes and a dosimeter then manages the collection of Rubidium-82 within the CardioGen-82 device, by using a saline solution. The saline is eventually sent through the tube, and it collects the Rubidium, while attempting to avoid collecting the more radioactive Strontium-82.
If Strontium-82 manages to slip into the final solution in a greater than permissible (trace) amount, that contamination is termed “breakthrough.” Obviously, breakthrough is not permissible because the highly radioactive Strontium-82, having a half-life over 1,000 times as much as Rubidium, might cause unwanted health risks for a patient undergoing a diagnostic cardiac PET scan.
As mentioned, very few facilities in the world can make enough Strontium-82 to manufacture the Strontium-Rubidium generator. As a result, Bracco Diagnostics, Inc. partnered with MDS-Nordion to develop a new dedicated manufacturing facility in Ottawa, Canada to produce CardioGen-82.
Yet, even before the Bracco-Nordion partnership, the Canadian Government and the AECL (Atomic Energy of Canada) had “walked off the job” by cancelling a commercial contract for which they had already paid $350 million dollars to complete. The Canadian Government did so without disclosing any alternative plan to produce isotopes for world consumption, except to relicense an already existing and decaying 50-year old NRU (National Research Universal) facility. That decision was a blow to worldwide isotope supply stability, and it angered leaders in the nuclear medical field who had not advocated local manufacture in their own countries, because they were waiting for Canada to bring the new Maple Reactor Project (MAPLEs) facility on line.
In 2009, the older Canadian facility (the NRU) was one of five reactors in the world capable of producing large quantities of medical isotopes. The second largest producer was the Petten reactor in the Netherlands, which manufactured 30% of the world’s supply, and it had to shut down due to a water leak. As a result, there was a strontium-82 shortage as early as 2009.
According to a company report, MDS Nordion began manufacturing CardioGen-82 for Bracco Diagnostics in June 2009. Prior to the Bracco-Nordion partnership, Los Alamos National Laboratories had generated Strontium-82 in dedicated runs and sent the material to General Electric Healthcare for loading into CardioGen-82 generators.

Again in 2009, Bracco released a letter indicating that PET reimbursement was going to increase by 24%. Of course, the increased reimbursement for PET diagnostic procedures would generate a greater demand for the procedure and further tax the already short supplies of Strontium-82.
Proceeding along, in 2011, a Nordion report pointed out that Bracco Diagnostics, Inc. was going through a manufacturing process change with a third party supplier, relating to component modifications. As a result, Nordion had not been manufacturing CardioGen generators since Quarter 2 of 2011. In that report, Nordion also stated that it was working with Bracco to resolve the issue as soon as possible and to resume manufacturing activities.
Therefore, a simultaneous isotope shortage and third party component supply problem was occurring in mid-2011. Reportedly, the isotope shortage led Bracco Diagnostics to attempt to increase the life of its generator from 28 days to 42 days, and the FDA “was on board with this”.
In a May 5, 2011 letter to Valued Customers, Bracco Diagnostics stated the following:
“As you know, we are in the middle of our Sr-82 raw material supply limitations. During this period, we are focusing on providing you with timely information and routine generator scheduling updates. We understand you may have modified your cardiac PET program to align with optimizing generator utility. An ‘Extended Use Generator Program’ will also be rolled out later this year, offering a six week delivery schedule for customers that will benefit from this program.
Recently, we communicated generator delivery schedule changes due to an unexpected manufacturing process issue. Customers are being notified routinely with definitive generator availability information. Moving forward, we anticipate finalizing production enhancements over the next few months and providing you, our Cardiogen-82 customers, with your respective Cardiogen-82 delivery schedule for the remainder of the year. Your Bracco Nuclear Medicine Accounts Manager will continue to keep you apprised of the situation.”
“Based on further investigation, FDA has determined that the current CardioGen-82 manufacturing procedures are not sufficient to ensure reliable performance of the generator used to produce the Rb-82 chloride injection.”.
Presently, it remains clear that there is no news yet available that would give a precise picture of exactly what caused the suspected Strontium breakthrough to occur and to cause certain CardioGen-82 patients to become walking repositories of radioactivity. Nevertheless, Bracco has already announced that it will be reintroducing CardioGen-82 to the market in the first half of 2012:
“Bracco has proposed an orderly, phased reintroduction of CardioGen-82 generators to user facilities, with data collection and evaluation of the actual field use of each generator. Based on the outcome of our reintroduction discussions with the FDA, Bracco anticipates a limited and progressive reintroduction of the product to commence in the 1st or 2nd quarter of next year.
Should the FDA contact your site to request information regarding your use of CardioGen-82, your cooperation with the agency’s information-gathering efforts will help support the market return of CardioGen-82? It is important for our customers and the professional societies to communicate to the FDA their experiences with CardioGen-82 and its utility in PET myocardial perfusion studies.
We know these circumstances have seriously affected our customer sites, and we appreciate the loyalty and support of our customers toward the closure of the current voluntary recall and the expeditious market return of CardioGen-82.”.
Given this news, perhaps the FDA should shed at least a shred of light, concerning the reasons why CardioGen failed in the first place.
Certainly, legitimate and important questions are raised in this situation:
- Was it because the isotopes were rushed to market and were not sufficiently pure to begin with?
- Was it because CardioGen procedures are too complicated and personnel need special training and more oversight?
- Could the instrument itself be too complicated to maintain and/or too technique sensitive?
- Was the suspected breakthrough of Strontium-82 due to Bracco extending the number of days the CardioGen could be used from 24 to 42 days (end of cycle use increases the possibility of Strontium-82 breakthrough)?
- Was it the generator itself that was not manufactured to specs? Did different suppliers provide less pure starting materials?
- Was something inherent in Nordion’s CardioGen-82 manufacturing process that caused the problem?
- Was an inherent CardioGen design defect responsible for the failure?
As of October 22, 2011, after hours of scouring resources, there are yet no definitive published answers to these questions. However we do know that Bracco is planning reintroduction of CardioGen within months. Something seems to be missing from this picture — perhaps responsible manufacturing or oversight?
For additional references, please refer to:
http://www.nordion.com/documents/Opening_remarks_Natural_Resources_Committee_11Jun09.pdf
http://www.nordion.com/documents/Medical_Isotope_Supply.pdf
http://www.nordion.com/reports/2010_engaif.pdf
http://www.nordion.com/reports/2010_engannual.pdf
http://www.nordion.com/reports/2011_engq1.pdf
http://www.fda.gov/Drugs/DrugSafety/ucm265278.htm
http://www.dotmed.com/legal/print/story.html?nid=17155
Share This


