Cook PTX Stents Issued Again After Voluntary Recall
Cook Medical is once again shipping the Zilver PTX Drug-Eluting Peripheral Stent to doctors and hospitals in Europe, Japan and in the U.S. following a global recall due to at least one fatality.
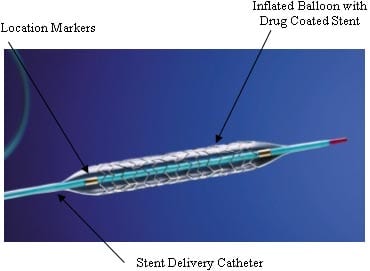
The stent was voluntarily recalled after the company received 13 complaints of a faulty delivery system. Specifically, the tip separated at the time of implant which resulted in two complications and one death. Further complications can include thrombosis, amputation and possible cardiac arrest.
In April, the privately-held medical device company based in Bloomington, Indiana issued the recall just a few months after the FDA gave it its approval for use in the U.S. The recall affected devices made from December 1, 2012 through April 16, 2013 and did not include the non-drug eluting bare-metal version of the stent.
Since April, the recalled device delivery system has been amended by Cook.
The company said the device had separated internally from its delivery catheter during several implantations, leading to the Class I recall, the most serious issued by the FDA. A Class I recall means the device has the potential for serious injury or death. Consumers are asked to provide any adverse reports through the FDA’s MedWatch program online or by phone.
When it was approved in the U.S. in November 2012, the Zilver PTX became the first drug-eluting stent used for treatment of peripheral artery disease (PAD), a condition where blood flow to extremities such as the legs is blocked due to narrowed arteries.
The device is self-expanding and is inserted in the femoral artery to emit the inflammation-reducing drug, paclitaxel, used to prevent restenosis.
The British medical journal The Lancet reports rates of PAD are now at an epidemic level at 38 million patients worldwide.
Profits per stent were $2,750, according to Millennium Research Group, and the device was targeted to generate about $100 million in annual revenue before the setback.
The recall relates to the delivery system and not the stent, which is thought not to pose any problems in the 30,000 patients worldwide who have received it.
Share This