The AMS Mesh Litigation
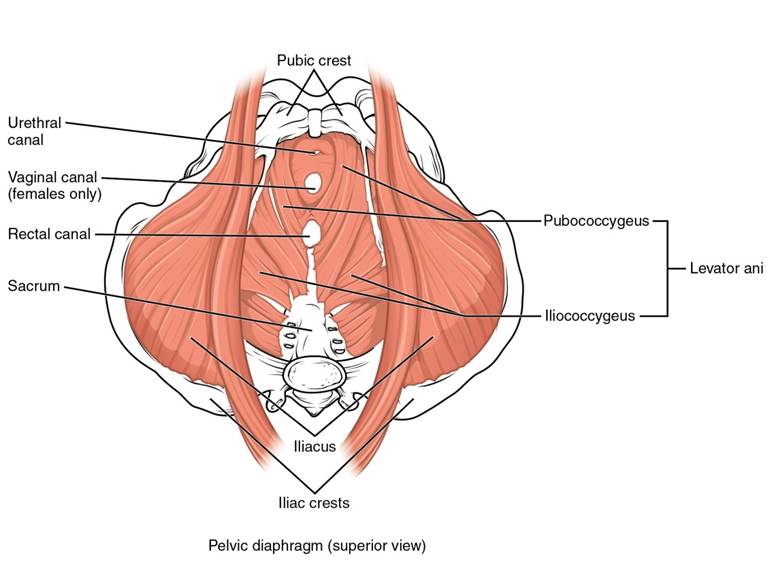
Since transvaginal mesh was first introduced into the market over 20 years ago, many hundreds of thousands of women have undergone operations where doctors have inserted vaginal mesh devices, threading them through incisions in the vagina to fortify pelvic muscles that failed to support internal organs or to treat incontinence, according to court filings.
All filed federal product liability lawsuits claiming injuries with transvaginal mesh or bladder slings used for repair of pelvic organ prolapse and female stress urinary incontinence are centralized. In February 2012, multidistrict litigation (MDL) cases against the mesh manufacturers were consolidated. The Ethicon consolidated cases are In re Ethicon Inc. Pelvic Repair System Products Liability Litigation.
Since 2008, thousands of mesh cases were filed alleging that American Medical Systems products, which were implanted to treat stress urinary incontinence and pelvic organ prolapse, led to complications.
Since transvaginal mesh was first introduced into the market over 20 years ago, millions of women have undergone the implantation procedure. In 2010 alone, surgeons around the country inserted over 70,000 of these mesh devices. The surgeons typically thread the product through incisions in the vagina to fortify pelvic muscles that failed to support internal organs or to treat incontinence, according to court filings.
All filed federal product liability lawsuits claiming injuries with transvaginal mesh or bladder slings used for repair of pelvic organ prolapse and female stress urinary incontinence are centralized. In February 2012, multidistrict litigation (MDL) cases against the mesh manufacturers began. This legal process streamlines hundreds of individual claims against the makers of the medical devices. The United States District Court for the Southern District of West Virginia is the site of the coordinated litigation for transvaginal pelvic mesh lawsuits. There are approximately 70,000 plus cases against different manufacturers in the MDL. Judge Goodwin is the federal judge who oversees the consolidation of the transvaginal mesh cases in Charleston, West Virginia, which include American Medical Systems. The American Medical Systems consolidated cases are In re American Medical System Inc. Pelvic Repair System Products Liability Litigation.
Defendant’s Pelvic Mesh Products that are listed in American Medical System master complaint are as follows:
- Apogee
- Perigee
- MiniArc Sling
- Monarc Subfascial Hammock
- SPARC
- In-Fast
- BioArc
- Elevate
- Straight-In
American Medical Systems, a unit of Endo Pharmaceuticals, agreed to settle an unspecified number of claims for pelvic mesh products for $54.4 million. The settlement agreement involved lawsuits filed in both state and federal courts and was reached in June 2013, just a month after American Medical Systems estimated its liability at $159.8 million. American Medical Systems was among several companies the Food and Drug Administration (FDA) ordered to conduct post-market studies of surgical mesh in 2012. It is estimated that the average award per plaintiff will be around $40,000.
Bellwether trials have been used in our legal system where there are large numbers of similar claims or lawsuits against the same defendant. A bellwether trial is a procedure where a representative case or cases are selected to be tried before a jury to assist the court and the parties in evaluating information and evidence, and possibly predicting future trends about a larger group of cases. Judge Goodwin is overseeing the bellwether trials.
In July 2013, Judge Goodwin had selected the following bellwether cases for trial: Fontes v. American Medical Systems, Inc., 2:12-cv-02472, Serrano v. American Medical Systems, Inc., 2:12-cv-03719, Jilovec v. American Medical Systems, Inc., 2:12-cv-05561, and Weiler v. American Medical Systems, Inc., 2:12-cv-05836. The trials were set to commence in June and July of 2014, however, the parties utilized the period prior to trial to negotiate terms of a settlement.
As of April 15, 2014, the Judicial Panel on Multidistrict Litigation reported over 16,000 American Medical Systems mesh cases alone coordinated in federal court under U.S. District Judge Joseph Goodwin in the Southern District of West Virginia.
In May 2014, American Medical Systems reached another agreement with multiple plaintiffs’ to settle approximately 20,000 mesh claims for up to $830 million. The total amount paid will ultimately depend on the number of claimants who participate in the settlement. If less than all of the claimants participate, the total settlement amount will be reduced by an amount for each non-participating claim agreed to by the parties.
The news of this resolution was published on the heels of the FDA issuing two proposed orders to address the health risks associated with transvaginal mesh used to repair pelvic organ prolapse, or the weakening of a woman’s bladder, uterus and bowel after childbirth, aging or other causes. According to the FDA’s news release, if finalized, “the orders would reclassify surgical mesh for transvaginal pelvic organ prolapse from a moderate-risk device to a high-risk device and require manufacturers to submit a premarket approval application for the agency to evaluate safety and effectiveness.”
Share This