Levaquin and the Status of Multi-District Litigation
On August 10, 2011, Judge John R. Tunheim, District Court Judge in Minneapolis, held a Joint Status Conference for the coordinated mass torts proceeding involving the drug Levaquin. Currently, there are 1,314 individual lawsuits that have been transferred to MDL No. 1943 for pre-trial proceedings. Judge Tunheim has completed two bellwether trials in these multi-district litigation proceedings, which were established in June of 2008. There are also 1,831 cases pending in coordinated proceedings before Judge Higbee in state court in New Jersey, 39 cases in various Illinois state courts, and 6 cases in other state courts around the country.
The first trial in the MDL was completed in December of 2010. In this case, a Minnesota federal court jury returned a verdict in the amount of $1.8 million against Johnson & Johnson and its subsidiary Ortho-McNeil Pharmaceutical, Inc. after a three-week trial. The verdict included $700,000 in compensatory damages for an 82-year old man who suffered an Achilles tendon rupture in 2005. The jury also awarded $1.1 million in punitive damages, as it found that Johnson & Johnson and Ortho-McNeil had acted with a reckless disregard for the safety of the plaintiff and others in failing to warn that Levaquin was more likely to cause tendon ruptures than other antibiotics with similar efficacy. The damages against the manufacturers of Levaquin will be reduced because the jury allocated 10% of the liability to the plaintiff and 15% to the prescribing physician.
The second jury trial in the MDL bellwether trial process was completed in June of 2011, and ended with a verdict in favor of the drug manufacturers. This case involved a Minnesota man who had to undergo surgery after his Achilles tendon ruptured within days of his use of Levaquin for pneumonia.
The next trial against the manufacturers of Levaquin is scheduled to begin on September 6, 2011, in the New Jersey state court proceedings, and is expected to last for several weeks. The third jury trial before Judge Tunheim in the MDL is slated to begin on November 14, 2011, and the individual Minnesota plaintiff whose case will be tried at that time should be selected shortly.
Judge Tunheim indicated at the recent hearing that he would revisit requests to activate broader discovery (including depositions of plaintiffs, treating physicians, prescribing physicians, and surgeons) in several months once the upcoming trials are completed. Requests by plaintiffs to remand or transfer the remaining cases in the MDL back to local jurisdictions for individual trial settings will likely be heard at that time as well.
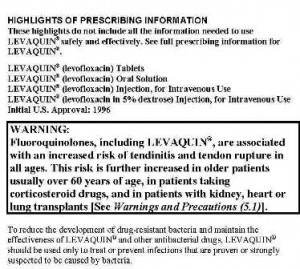
Levaquin is a very popular antibiotic that is part of the fluoroquinolone family. It remains on the market today, despite being linked to thousands of serious injuries. Unfortunately, some elderly patients who have suffered tendon injuries have been unable to undergo successful surgical repair of their tendon ruptures, and are now wheelchair-bound or have serious difficulty with ambulation.
Share This


