FDA Fails in Many Drug and Device Reviews
A trio of studies published in the Journal of the American Medical Association (JAMA) finds that just because a drug or medical device makes it to market does not necessarily mean it’s been tested and is safe and effective beyond all doubt.
According to three published reviews, the same standards are not used to review drugs. Clinical trials may vary from one drug to the next. Early problems that arise in drug trials are often overlooked until after a drug is marketed and those problems appear in real life and we know from the debacle that is hip prosthesis and transvaginal mesh that clinical trials are not required at all for most medical devices.
The problem is pressure from the manufacturers who want to get their products to market quickly with the least amount of resistance from the watchdog agency. Even those within the agency who want to protect the public often find themselves outvoted by the push to market.
One published review from Yale University reveals about one-third of drug approvals were based on one trial only. Incredibly, about ten percent of drug trials did not compare the medication to another one or a placebo.
And there is no consistency in clinical trials. The number of enrollees varied as did the duration of the trial. In the end the public, doctors and patients have no idea what evidence was considered before a drug is approved.
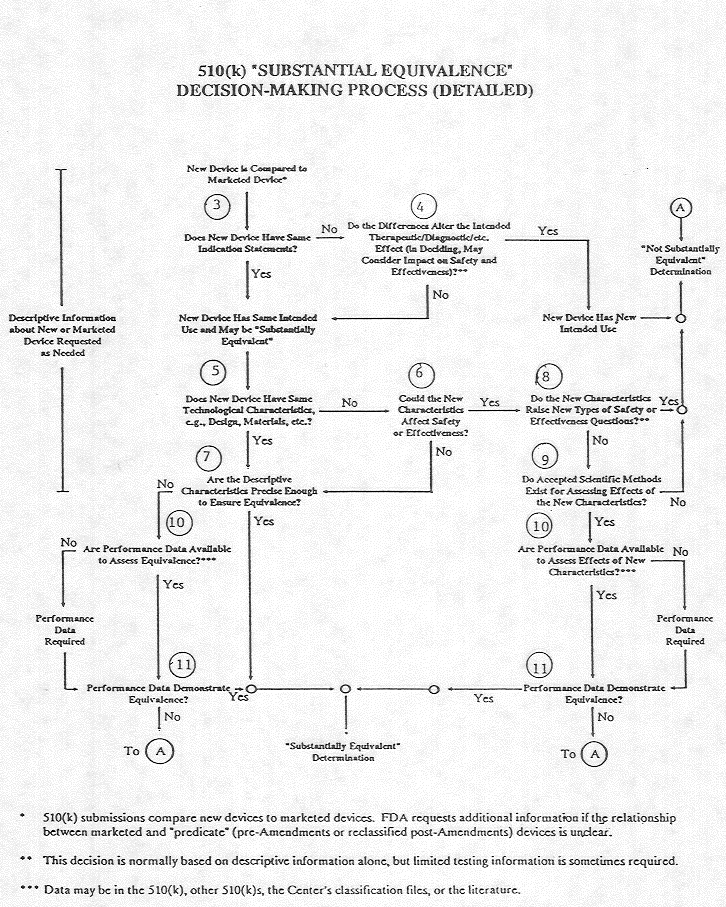
We already know that the majority of medical devices such as defibrillators and pacemakers, transvaginal mesh and hip prosthesis are approved based on an earlier model that is already being sold. Since 1979 there have been 5,829 device approvals that made it market based on being the “substantial equivalent” to another being sold even though the new device may have a different design or material.
Even with changes and even for permanently implanted medical device, a clinical trial was not required. The end result – life-threatening devices can fall between the cracks and make it to market. Devices such as the DePuy ASR hip, six makers of defective transvaginal mesh, the Medtronic Sprint Fidelis defibrillator leads and the St. Jude Medical Riata are examples of medical devices that were approved this way.
Share This