Consumer Reports: Dangerous Medical Devices Approved Through Flawed Approval Process
We hear from them every day – consumers who trust and believe their doctor only to find out things like what Consumer Reports (CR) investigates in its May issue. Surprisingly, the majority of medical devices never have to pass through any sort of safety and efficacy standard with the Food and Drug Administration (FDA) – the agency we rely on to keep us safe!
The more the public finds out about this huge regulatory loophole the more people will speak out and the faster something will be done. At the current time, the loophole in regulation exists at the expense of public health.
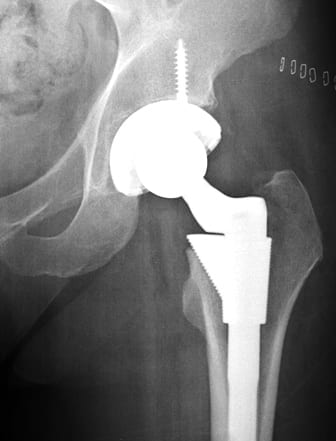 Millions of Americans live their daily lives with an artificial hip, knee, heart defibrillators or surgical mesh implanted in their body. CR reminds us that most of these devices made it onto the market after a $4,000 investment by the device maker and an exchange of paperwork with the FDA. To obtain approval, an FDA regulation known as 510(k) only requires a device maker to name an older device already being sold and show the new device is “substantially equivalent” to the older device.. Device makers get very creative in how they interpret the term “substantially equivalent.”
Millions of Americans live their daily lives with an artificial hip, knee, heart defibrillators or surgical mesh implanted in their body. CR reminds us that most of these devices made it onto the market after a $4,000 investment by the device maker and an exchange of paperwork with the FDA. To obtain approval, an FDA regulation known as 510(k) only requires a device maker to name an older device already being sold and show the new device is “substantially equivalent” to the older device.. Device makers get very creative in how they interpret the term “substantially equivalent.”
It doesn’t matter if the device is novel in its engineering, its materials or its science. Truth be told, the manufacturer doesn’t even have to be currently able to make the device!
Ninety percent of medical devices are approved this way, a far cry from the much more stringent premarket approval that a new drug must undergo.
You may recall that back in July 2011, the Institute of Medicine issued a special report that said the 510(k) system was “fatally flawed” and should be overhauled. Since that time just the opposite has occurred and the FDA is moving in the direction of making medical device approval even faster. Device manufacturers continue to lobby for passage of the Medical Device User Fee Act which would grease the skids for even speedier approval.
Since device manufacturers are not required to submit safety or efficacy studies before a product is approved for sale, most have little if any idea how the devices will actually perform when implanted in human beings. “What they’re doing is conducting clinical trials on the American public,” says Dan Walter, whose wife suffered with a heart catheter that malfunctioned.
Others mentioned in the report are Janet Holt, who was implanted with synthetic surgical mesh for a prolapsed uterus and bladder. Eight removal surgeries later she’s been left with permanent nerve damage and says the mesh has “ruined my life.”
The FDA is considering reclassifying synthetic surgical mesh, now considered of moderate risk, to a higher risk Class III device. In the meantime, it is still on the market and about 100,000 are being implanted every year as a treatment for pelvic organ prolapse and incontinence.
Lisa Wilson, 46, had a Lap-Band procedure after many unsuccessful tries to lose weight. But the band cut into her stomach lining and sent her to the hospital with an infection for eight days. An estimated 650,000 Lap-Bands have been sold and one study shows 51 percent of patients had complications and 25 percent had their bands removed.
Consumer Reports cites the Journal of the American Medical Association report that found only 27 percent of Lap-Bands sold were involved in randomized clinical trials.
Stephen Tower, M.D., an orthopedic surgeon from Anchorage, Alaska received a DePuy artificial hip made by Johnson & Johnson. The ASR XL was made of chrome and cobalt metal and it was cleared for market after the device maker claimed it was “substantially equivalent” to another device already on the market. He started experiencing odd symptoms, the cause of which he and his doctors could not figure out. After finding his blood contained dangerous levels of chromium and cobalt he had the hip removed. His symptoms markedly improved.
If a patient experiences an adverse reaction while taking a drug, the patient can stop taking it. In the case of an implanted medical device however, removing the device may be impossible or impracticable, requires repeat surgery and all the risks that entails and is expensive.
Consumer Reports suggests the system is broken and needs to be fixed. The advocacy arm of Consumer Reports says we need to:
- Require premarket testing, as drugs undergo, for safety and efficacy
- End the grandfathering in of high-risk new implants
- Give implants an ID number so a patient can be notified if there is a problem or a recall
- Create a national registry of patients and their implants
- Increase the user fees paid by manufacturers to the FDA so the agency can hire more reviewers to handle the load of new device applications
Otherwise it is the public who is undergoing the real-life clinical trials as guinea pigs, and most people don’t even know it.
Share This


