C.R. Bard Awarded Summary Judgment on Warning Claim in IVC Case
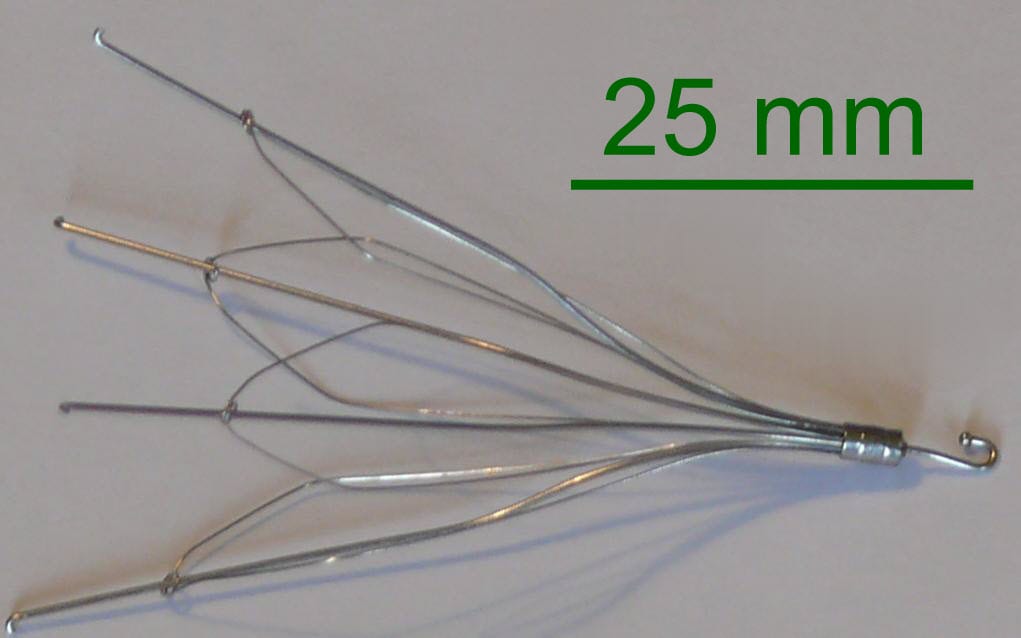
An inferior vena cava (IVC) filter is used to prevent life threatening pulmonary emboli. Once installed, the filter’s arms and legs open and anchors it to the walls of the IVC. The filter then catches blood clots that would otherwise flow into the heart and lungs as pulmonary emboli.
The plaintiff at issue was implanted with a G2X IVC filter, which is manufactured by C.R. Bard (Bard). She and her husband sued Bard, alleging that she suffered and continues to suffer severe medical consequences as a result of the damage caused to her by the filter. They asserted claims of loss of consortium, negligence, negligent misrepresentation and strict products liability based on the IVC filter’s design defects, failure to warn, and manufacturing defect. Plaintiffs seek compensatory and punitive damages.
Bard moved for summary judgment on all claims. Judge Honeywell of the United States District Court, Middle District of Florida agreed with Bard that the failure to warn claim failed because plaintiffs failed to proffer evidence that the G2 filter’s Instruction For Use (IFU) failed to adequately tell the implanting surgeon of the device’s risks, including its risk for perforation.
The judge held that plaintiffs have failed to provide any admissible expert testimony that would establish the inadequacy of the IFU. In her opinion, she stated, “Further, although Plaintiffs note that Dr. Zweibel testified that he would have liked to have comparative failure rates, and that such information would have been helpful to him in weighing the risks and benefits of the device, this testimony establishes, at most, only that additional information would have given him better warning, but fails to establish that the IFU was actually inadequate in informing him of the risks of the G2 filter.”
The judge rejected plaintiffs’ arguments that because the perforation arouse out of the normal use of the G2 filter, they are entitled to an inference of manufacturing defect. However, the Judge noted that the evidence is undisputed that perforation is a risk inherent in the design of all IVC filters, including the design of all IVC filters. Therefore, the Judge held that the mere fact that the plaintiff perforated her IVC is insufficient to establish that it malfunctioned.
The judge awarded Bard summary judgment on the manufacturer defect and negligent misrepresentation claims, finding that plaintiffs failed to present evidence that the filter varied from other filters and that Bard made misrepresentations to the implanting surgeon.
However, the judge allowed the design defect and punitive damages claims to proceed, finding the plaintiffs presented evidence that the G2’s overall design was unreasonably dangerous and that Bard knew the device could injure the plaintiff.
Share This