Antibiotic Use Rule Changes Proposed for Livestock Industry
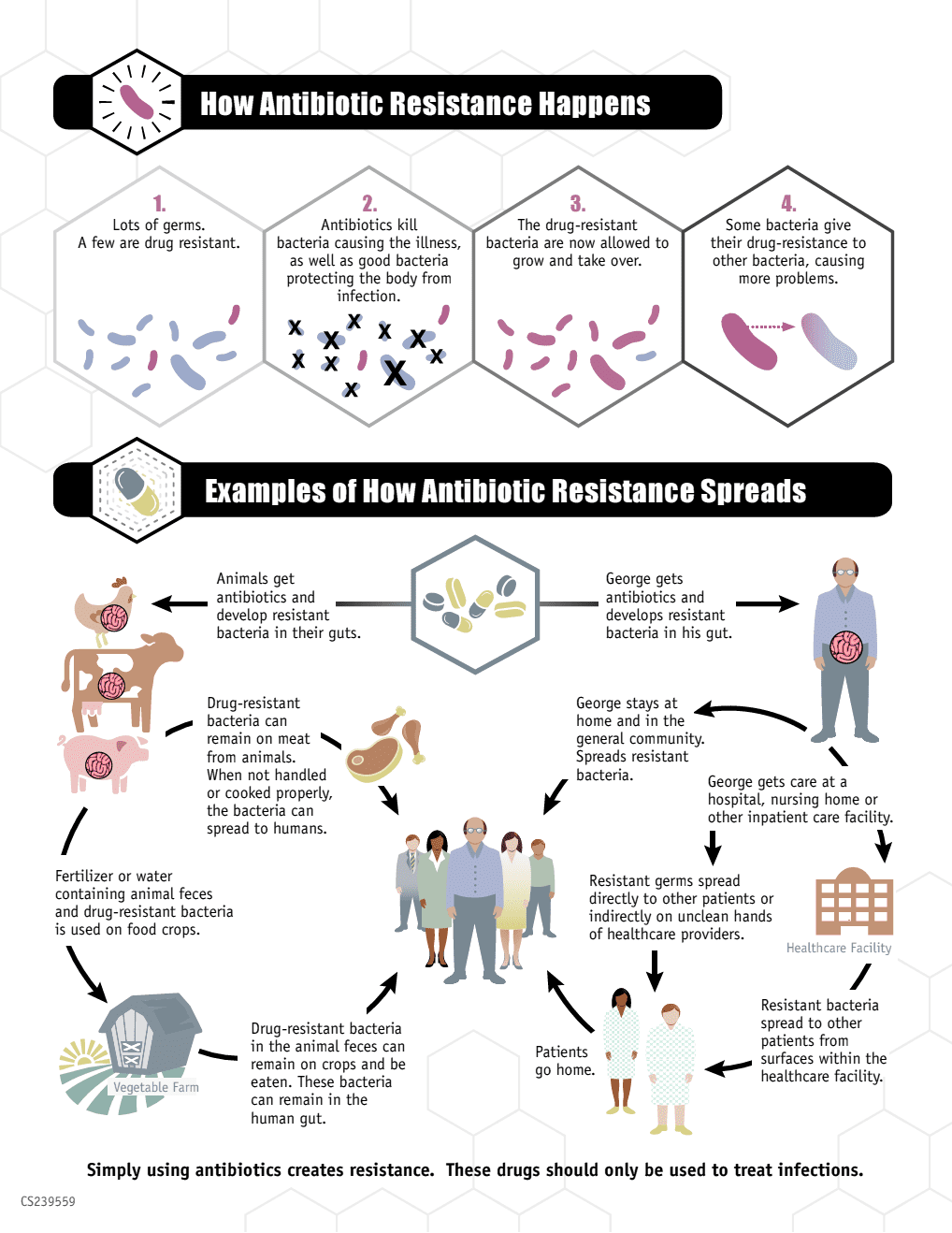
CDC infographic detailing the dangers of overuse of antibiotics in people and livestock.
The U.S Food and Drug Administration (FDA) is proposing a ban on the routine use of antibiotics as a growth promoter in animals raised to be consumed by humans. Because of cramped and dirty conditions present in factory farms, animals are raised in a less than ideal circumstance. A low dose of antibiotics is delivered in the animal’s food to ward off disease, keep profits up and to promote animal growth, though no one knows why that happens.
Most industrial nations long ago banned the use of antibiotics in the animals that produce the food we consume because when they are needed to fight disease in humans, the same antibiotics are less effective and are thought to contribute to antibiotic-resistant disease. Bacterial diseases evolve quickly in the presence of antibiotics to evolve a resistance to them.
There are about 23,000 deaths from antibiotic-resistant infections every year in the U.S., reports the Centers for Disease Control and Prevention (CDC), and millions more fall sick. There have been warnings for the past 45 years that the overuse of antibiotics in our food would make antibiotics less effective when we need them but the powerful food lobby and its friends in Congress have stopped any effort to reduce their use.
Under the FDA changes, the use of certain antibiotics on livestock would have to be prescribed by a veterinarian by prescription only.
Criticism that the FDA regulations are utterly insufficient writes Scientific America in an opinion piece. The label change would only ask drug companies to voluntarily curb sales of drugs that are blended in food for agricultural use, drugs such as tetracycline, azithromycin and penicillins.
The FDA is asking drugmakers to change their labels making it illegal to use them for growth promotion. The label change could be phased in over three years before it would be illegal to use these drugs. Remember these are voluntary changes clearly showing who pulls the strings at the FDA and it isn’t public policy, safety and health. Drugmakers must tell the agency within 90 days if they plan to participate. Sounds like the tail wagging the dog doesn’t it?
So far drug makers Zoetis and Elanco said they intend to participate, reports the New York Times.
Many fear loopholes will allow livestock producers to claim they need to use the drugs, just as they had before, to keep animals from getting sick, not for growth promotion. However if the FDA ban went farther to ban their use for the prevention of disease, it would narrow the loophole and ultimately reduce the use of antibiotics on livestock.
Share This


