Another Report About Metal on Metal Joint Implant Failure
The evidence condemning Metal on Metal (MOM) implants continues to grow. In a recent announcement by the National Joint Registry for England and Wales (NJR), it was revealed that 30% of MOM implants manufactured by DePuy Orthopaedics, Inc., a division of Johnson & Johnson require removal and revision within six years of implantation. The industry standard is 15 years or more.
In its report, the NJR revealed that MOM hip devices experience “by far” the highest rate of revision including removal and replacement. NJR data indicates that these devices are failing at double or triple the rate of others at five years. In addition, the report indicates that as time passes, the failure rate increases when compared to other hip implants. The data also tell us that women fared worse than men.
This report follows DePuy and Johnson & Johnson’s recall of both its ASR XL Acetabular and ASR Hip Resurfacing systems in August of 2011. It appears the recall was warranted. At the time of the recall, the reported failure rate for these two systems was 13%. This new data suggests the failure rate was even higher at 17%. At six years the NJR reported that 29% or almost one third of these devices required revision. Even a lay person with no medical training should recognize that failure rate as staggering. It also calls into question how the company and regulators would fail to identify this problem before doctors started pounding these rods and cups into the hips of thousands of patients!
The sad truth is they didn’t. Even if the company did, it’s unclear what they would have done with the information. A DePuy “spokesperson” said in response to the reported 1/3 failure rate that it, “shouldn’t necessarily be taken as gospel.”
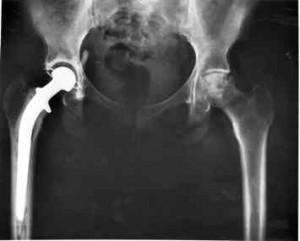 Unlike other consumer recalls, dealing with the problem is not as simple as taking your car into the dealership for repairs. These patients have the defective devices implanted in their bodies. Fixing the problem requires undergoing painful revision surgery. Doctors are on record saying that revision surgery is much more complicated than the original surgery and carries far more risks. Many are unsuccessful leaving the patient with permanent disabilities.
Unlike other consumer recalls, dealing with the problem is not as simple as taking your car into the dealership for repairs. These patients have the defective devices implanted in their bodies. Fixing the problem requires undergoing painful revision surgery. Doctors are on record saying that revision surgery is much more complicated than the original surgery and carries far more risks. Many are unsuccessful leaving the patient with permanent disabilities.
In addition to regulatory action in Europe, the FDA has ordered 21 manufacturers of MOM implants to carefully study the issue of whether patients with these implants are being poisoned by the release of metal shavings or debris that is cast off when the device fails. Studies of patients with MOM implants have reported abnormally high blood levels of Cobalt and Chromium which are potentially dangerous. The FDA is studying the long term effects of heavy metal poisoning in these patients.
Share This


